Risk Assessment, Comparative Analytics, and Trend Awareness
Post-Market Surveillance & Supply Chain Monitoring
Our Technology
All of our products are built on the same, automated, foundation: we gather, clean, analyze, and synthesize dozens of massive-scale data feeds using our patented Boomerang NLP™ technology. Boomerang NLP allows for the highly scalable natural language processing of diverse complex data; its accuracy has been validated in peer-reviewed journals.
Pharm3r’s technology platform can integrate both public and non-public data and handle structured, unstructured, and semi-structured datasets. This platform allows us to pool clean, usable data that can be piped into our main products.
Our Products
Our flagship software products include:
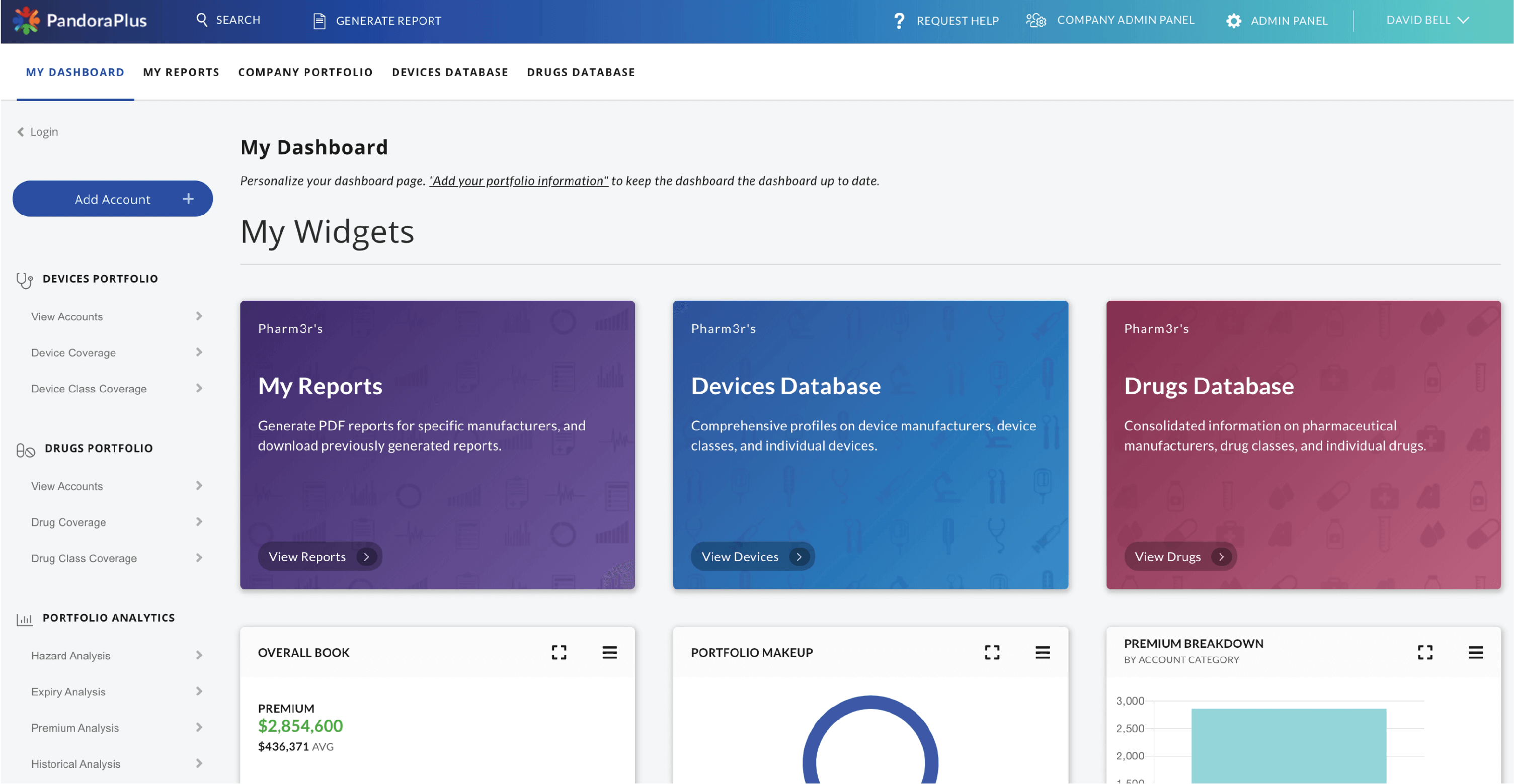
PandoraPlus™
Drug, device, and company information delivered via an intuitive web application
Designed as a comprehensive early risk warning system, PandoraPlus contains information on over 50,000 drug and device companies, including risk-ranked product lists (calculated using our litigation modeling algorithms), recalls, warning letters, inspections, clinical trials, import refusals, drug label changes, NDA ownership, and synthesized adverse event data and trends.
Before PandoraPlus
Teams would spend weeks and even months researching medical product risk and quality information. The data collected was often disparate, incomplete, and missing the pertinent relationships needed to make valuable insights that firms could trust.
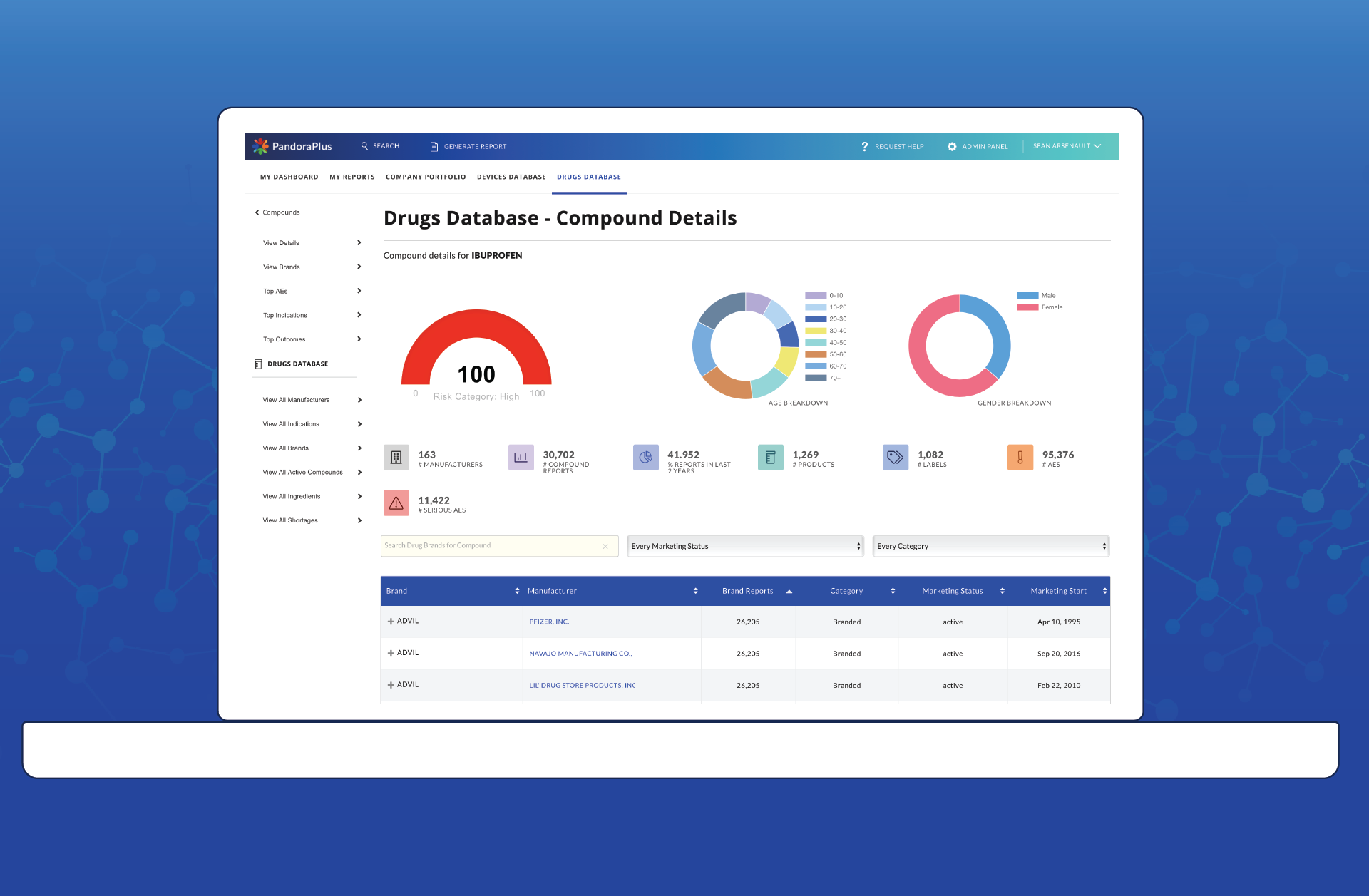
After PandoraPlus
At-a-glance risk assessment, historical trend analysis, prospective safety measures, and best-in-class comparisons make PandoraPlus the most powerful post-market surveillance tool available. Within minutes, customers are able to turn data assets into data insights, enabling them to make rapid and informed decisions.
QRx™
Identifies and evaluates drug supply chain relationships, quality metrics, and vulnerabilities
Containing data on thousands of companies, QRx provides information on their manufacturing facilities, including geographic locations and major products, as well as regulatory events linked to those facilities.
Clinical Trial Prospector™
Explores registered clinical trials, organized by sponsor and sponsor location
Our Clinical Trial Prospector helps customers monitor the dynamic drug and device market. Geographic overlays of trials can be customized, as well as views of patient profiles, exclusion criteria, and timelines. This tool is particularly useful for competitive intelligence and prospecting clients.
Portfolio Aggregator™
Reveals total exposure to a compound or device class by identifying products with heavily correlated risks
Our Portfolio Aggregator can incorporate limit, attachment point, premium, risk analysis, and benchmarking data into its visualizations, making it particularly helpful in scenario planning.
Medical Product Prospecting and Recommendation engine
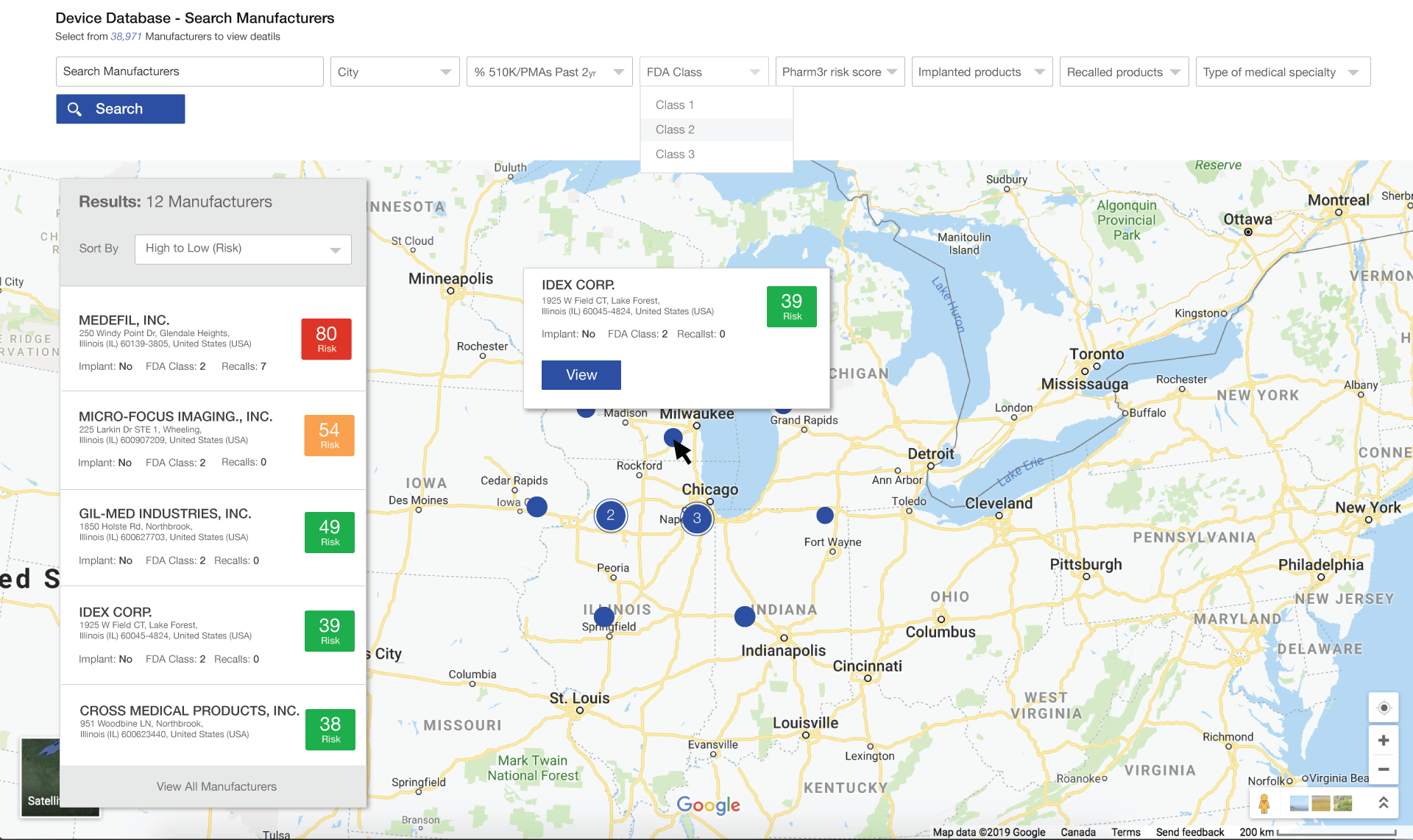
- Prospecting lists with custom filters for risk appetite driven product types, risk scores, company location
- Portfolio risk aggregation
- Recommendation engines
Vaccine analyzer
- Cleaned US and European vaccine adverse event data
- Adverse events mapped to common vocabulary for like comparison
- Disproportionality and trend analysis
